VALCHLOR® (mechlorethamine) gel is an alkylating drug indicated for the topical treatment of Stage IA and IB mycosis fungoides–type cutaneous T-cell lymphoma (MF-CTCL) in patients who have received prior skin-directed therapy
VALCHLOR—studied in the largest controlled trial of Stage IA/IB MF-CTCL1
VALCHLOR PIVOTAL STUDY DATA
The majority of patients responded to treatment with VALCHLOR1
Efficacy was demonstrated by 2 different response scales: CAILS (Composite Assessment of Index Lesion Severity) score and SWAT (Severity Weighted Assessment Tool). Patients were evaluated monthly for the first 6 months, then every 2 months until trial completion.1
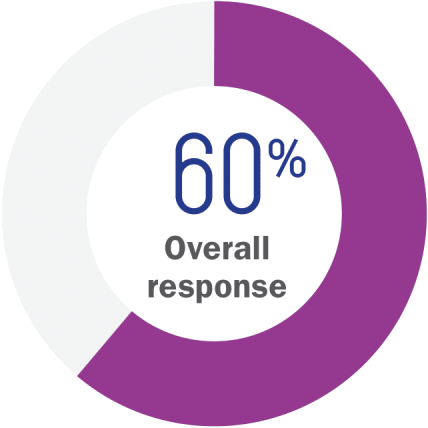
Primary endpoint—CAILS
60% overall response rate was achieved by patients receiving VALCHLOR1
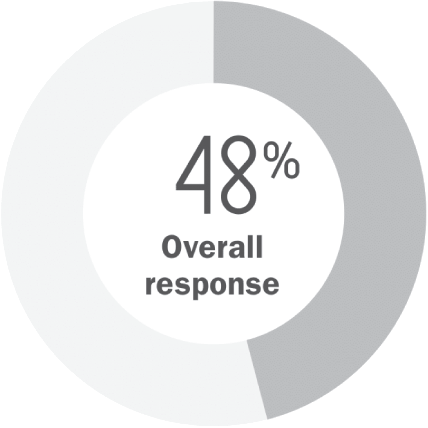
The primary study objective was noninferiority comparing VALCHLOR gel to compounded mechlorethamine based on a CAILS overall response rate. VALCHLOR gel was noninferior to compounded mechlorethamine based on the ORR ratio of 1.24 (95% CI 0.98, 1.58).1
VALCHLOR gel (n=119)
- Partial response: 45%
- Complete response: 14%
Compounded mechlorethamine (n=123)
- Partial response: 37%
- Complete response: 11%
Overall response defined as complete response plus partial response. Eighteen patients were excluded from both the CAILS and SWAT efficacy analysis due to protocol violations involving randomization at a single site.1
CAILS Score: Sum severity score of each of the following categories for 5 index lesions: Erythema, scaling, plaque elevation, and surface area1
Response: Partial response defined as ≥50% reduction from baseline CAILS score (confirmed after ≥4 weeks)1; complete response defined as a score of 01
Strong response was confirmed by SWAT score1
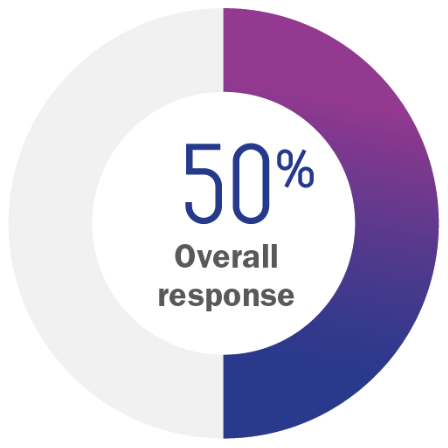
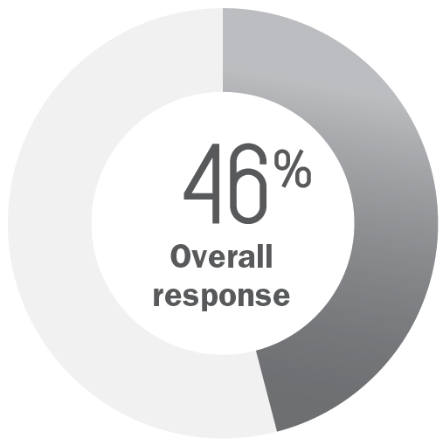
Secondary endpoint—SWAT
Half of patients receiving VALCHLOR achieved an overall response1
VALCHLOR gel (n=119)
- Partial response: 43%
- Complete response: 7%
Compounded mechlorethamine (n=123)
- Partial response: 43%
- Complete response: 3%
Overall response defined as complete response plus partial response.1
SWAT Score: Measuring each involved area as a percentage of total BSA, then multiplying it by a severity weighting factor: patch=1; plaque=2; tumor or ulcer=31
Response: Partial response was defined as ≥50% reduction from baseline SWAT score (confirmed after ≥4 weeks); complete response was defined as a score of 01, 2
Click below to hear an expert summarize key findings of the VALCHLOR pivotal trial
PIVOTAL STUDY DESIGN
VALCHLOR was assessed in a randomized, multicenter, observer-blinded, 12-month noninferiority trial of 260 patients1
Patients with MF-CTCL (N=260)
- Stage IA, IB, IIA* †
- ≥1 prior skin-directed therapy‡
- Median years of age
(gel: 57 years; ointment: 58 years)
Randomization (1:1)
VALCHLOR gel 0.016% w/w (Equivalent to 0.02% mechlorethamine HCl) once daily, n=130
Mechlorethamine HCl 0.02% ointment compounded in Aquaphor® once daily, n=130
Patients were stratified before randomization by Stage (IA vs IB and IIA).1
*Stage IA (58.5% gel; 50% ointment), IB (40% gel; 48.5% ointment).2
†While Stage IIA patients were included in the trial population, VALCHLOR is only indicated for Stage IA and IB MF-CTCL.1
‡Patients were not required to be refractory to or indolent of prior skin-directed therapies.1
Concomitant use of topical corticosteroids was not permitted during the study1
- Mean daily usage of VALCHLOR was 2.8 g (1 to 2 tubes per month); maximum daily usage was 10.5 g (5 to 6 tubes per month)1
| Baseline patient characteristics1,3 | VALCHLOR gel, % (n=130) |
Compounded mechlorethamine HCl, % (n=130) |
|---|---|---|
| BSA (body surface area) involvement, median | 8.5% (1% to 61%) | 9% (1% to 76%) |
| Received prior topical corticosteroids | 86.2% | 86.9% |
| Received prior phototherapy | 38.5% | 40.8% |
| Received Bexarotene (topical and oral) | 17.7% | 17.7% |
| Received topical mechlorethamine >2 years before study | 12.3% | 10.0% |
| Male gender | 59.2% | 59.2% |
| White race | 74.6% | 73.8% |
| Age 18 to 64 years | 71.5% | 66.2% |
ADDITIONAL DATA NOT CONTAINED IN THE USPI
CAILS time to response
Subgroup analysis of the primary endpoint: Efficacy Evaluable Population Group
- These data are from the pivotal trial, which was a noninferiority trial, and are not contained in the full Prescribing Information for VALCHLOR3
- These data should not be interpreted as providing better evidence of superiority or evidence that one treatment provides a faster or greater response than the other
Secondary endpoint—CAILS
VALCHLOR achieved a 50% CAILS response rate at 28 weeks3
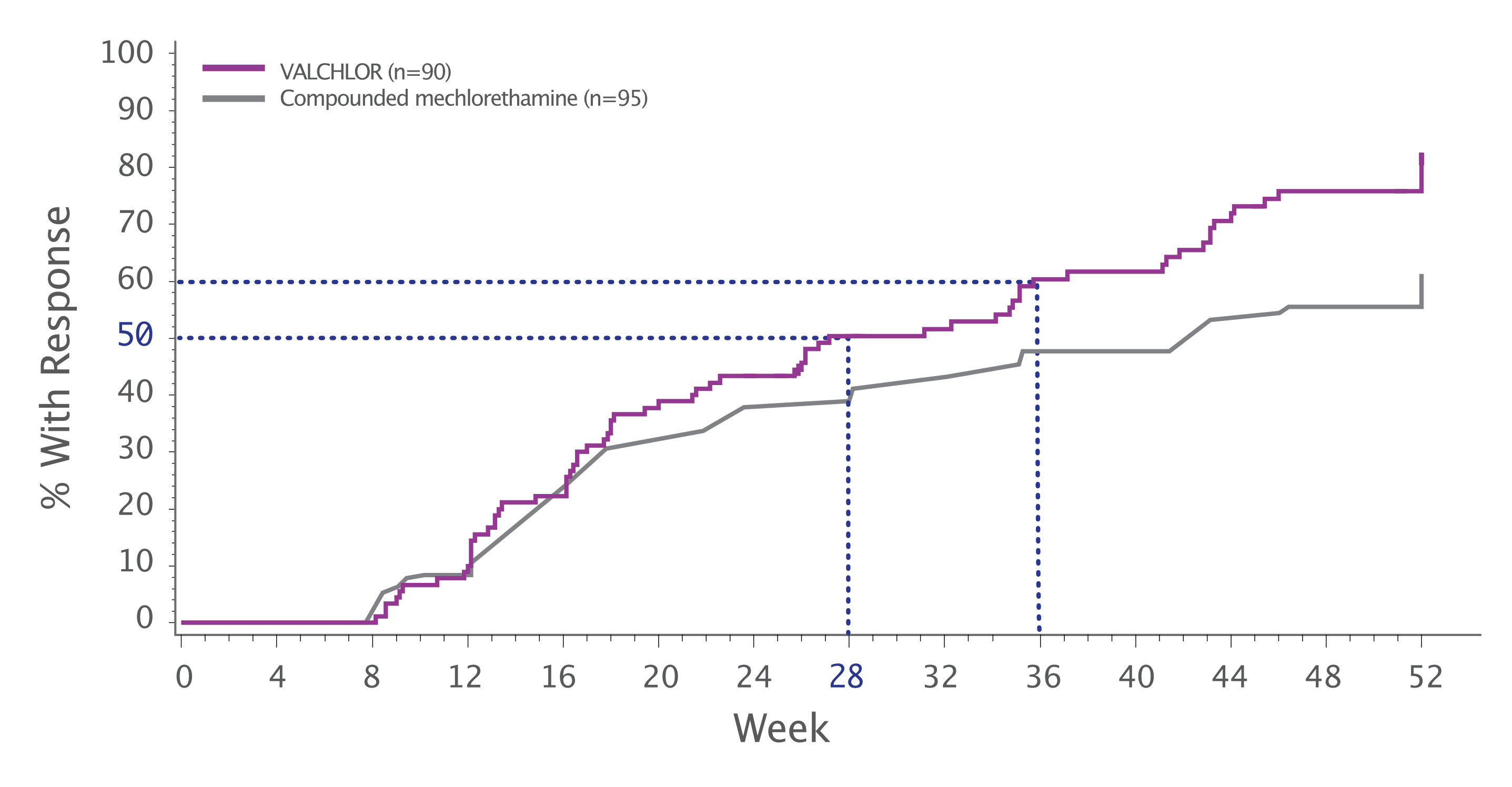
- The Efficacy Evaluable (EE) group is a subset analysis of the Intent to Treat (ITT) population group. EE patients received at least 6 months of study drug, had met the eligibility criteria, and had no significant protocol violations. A total of 72 (28.8%) of enrolled patients were excluded from the EE data set, 39 (15%) of enrolled patients were excluded due to withdrawal for skin toxicity prior to month 6. This is an expected side effect that typically occurs within the first few months of treatment3
- Time to first confirmed response could occur no sooner than week 8 per the study design3
Approximately 43% of patients receiving VALCHLOR responded at 24 weeks3
